- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >110611-21-7



Purity:99%
The synthesis of new tridental 2,4-bis(1...
The application of planar and central ch...
The reactions of benzaldehyde with dieth...
1,7,7-Trimethyl-3-(pyrid-2-ylmethyl)bicy...
An optically active zinc complex prepare...
Titanium(IV) complexes of bidentate tran...
Heterogeneous asymmetric addition of die...
A new optically active δ-aminoalcohol ha...
-
The optically active β-amino alcohol (1R...
A new bimetallic approach using dibenzof...
Various dialkoxides of zinc, magnesium a...
The C2-symmetric pyridine 1, incorporati...
Fermenting baker's yeast converts 4′-chl...
(Matrix Presented) The enantioselective ...
(S)-2-N,N-dibutylamino-3-butyl-1-[4-(4-p...
Polymer-bound ephedrine catalyzed the en...
Synthesis of diastereomerically pure o-h...
A chiral 2,2′-bipyridine ligand (1) bear...
We have demonstrated a green chemistry a...
Abstract: Chiral norephedrine-derived β-...
A new sterically hindered chiral P,N-lig...

1-(4-chlorophenyl)-1-propanol

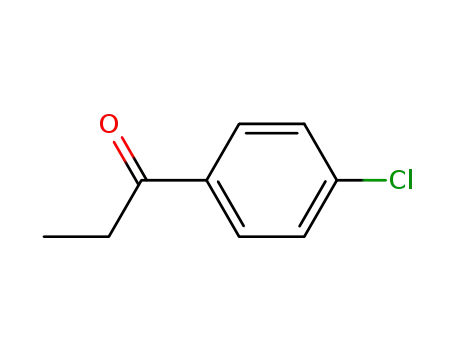
4'-chloropropiophenone

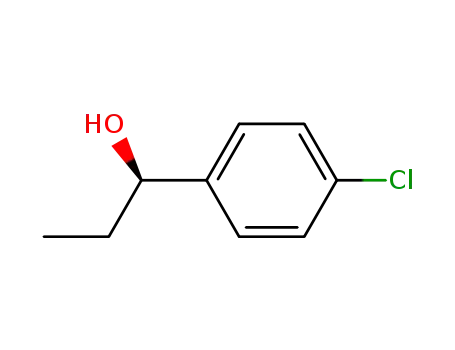
(R)-1-(4-chlorophenyl)-1-propanol
| Conditions | Yield |
|---|---|
|
With sulfuric acid; MnII((1R,2R)-N,N'-dimethyl-N,N'-bis((R)-(3,5-di-tert-butylphenyl)-2-pyridinylmethyl)cyclohexane-1,2-diamine)(OTf)2; dihydrogen peroxide; In water; acetonitrile; at 0 ℃; for 1h; Inert atmosphere; Schlenk technique;
|
90 % ee |
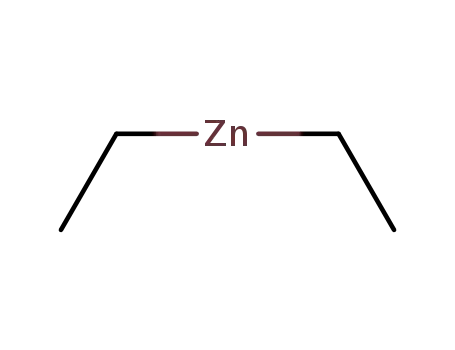
diethylzinc

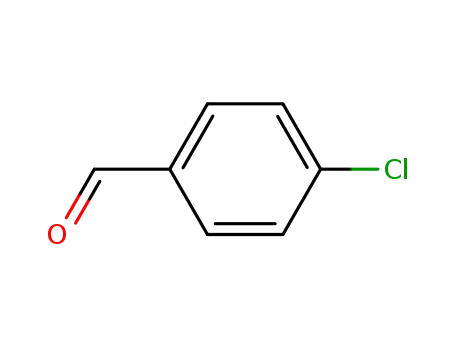
4-chlorobenzaldehyde


(R)-1-(4-chlorophenyl)-1-propanol


(S)-1-(4-chlorophenyl)-1-propanol

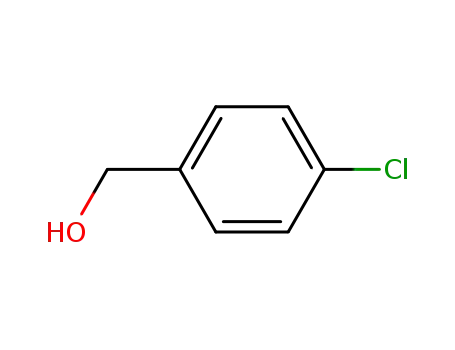
para-Chlorobenzyl alcohol
| Conditions | Yield |
|---|---|
|
With (1S)-(+)-3-exo-(dimethylamino)isoborneol; In toluene; at 0 ℃; for 12h; Yields of byproduct given. Title compound not separated from byproducts;
|
2% 86% |
|
With polymer-supported camphor derivative; In toluene; at 20 ℃; Title compound not separated from byproducts;
|
13% |
|
With (P,P)-(+)-bis[5]helicene diol; In toluene; for 24h; Title compound not separated from byproducts;
|
|
|
With chiral bicyclo[3.2.1]octane-based alcohol; In hexane; toluene; at 20 ℃; for 24h; Title compound not separated from byproducts;
|
|
|
With (-)-8-(9H-fluoren-9-ylidene)-1-(2-hydroxyphenyl)-7-methyl-5,6,7,8-tetrahydronaphthalen-2-ol; In hexane; toluene; at 0 ℃; for 168h; enantioselective reaction; Schlenk technique; Inert atmosphere;
|
35 % ee |
|
With (-)-8-(9H-fluoren-9-ylidene)-1-(2-hydroxyphenyl)-7-methyl-5,6,7,8-tetrahydronaphthalen-2-ol; In hexane; toluene; at 0 ℃; for 168h; enantioselective reaction; Schlenk technique; Inert atmosphere;
|
24 % ee |
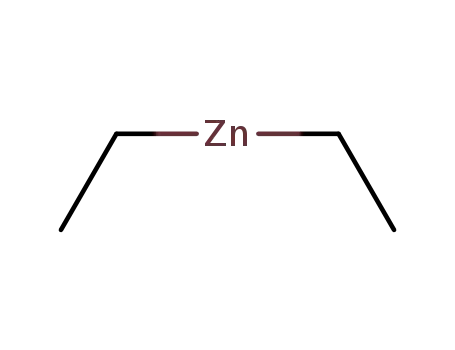
diethylzinc

4-chlorobenzaldehyde
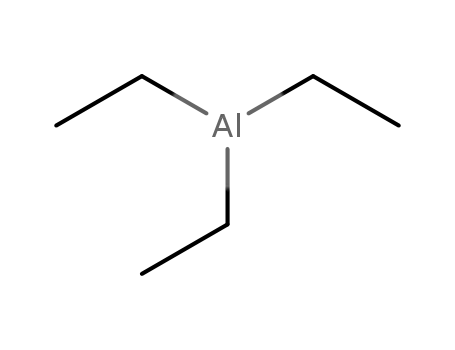
triethylaluminum
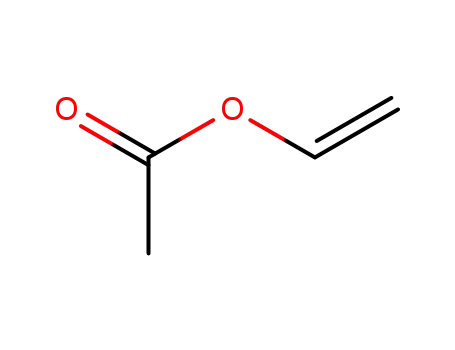
vinyl acetate