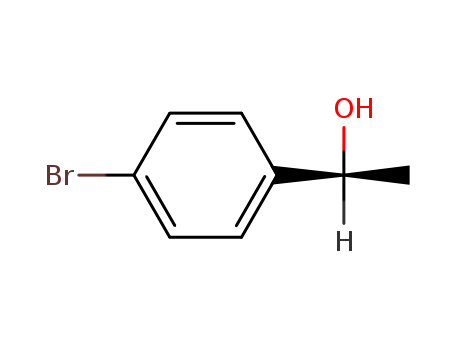
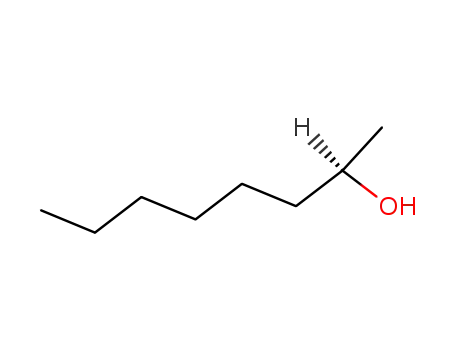
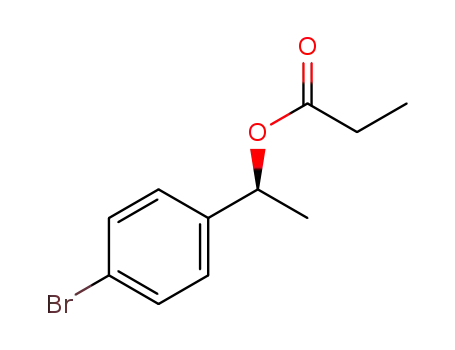
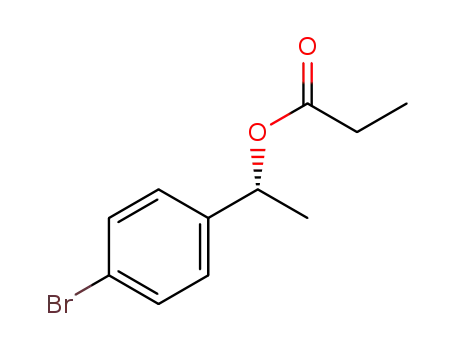
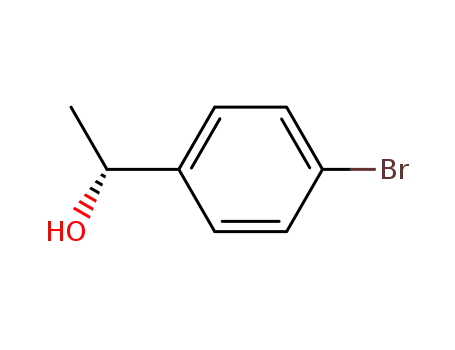
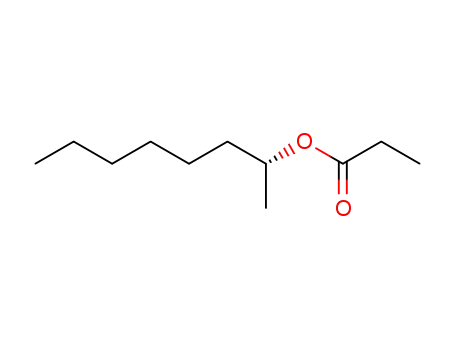
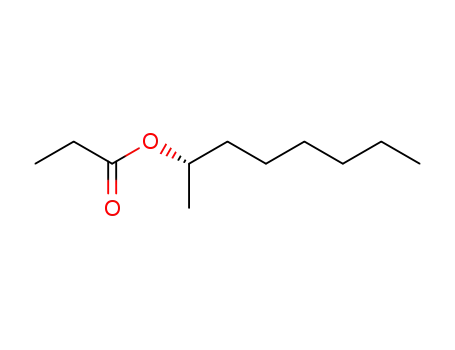
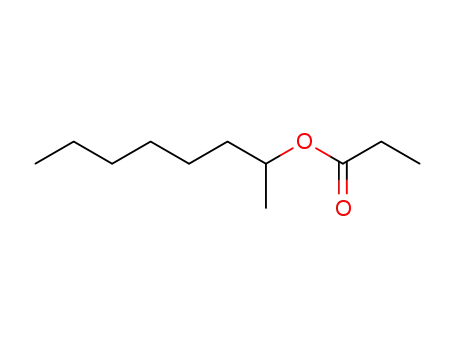
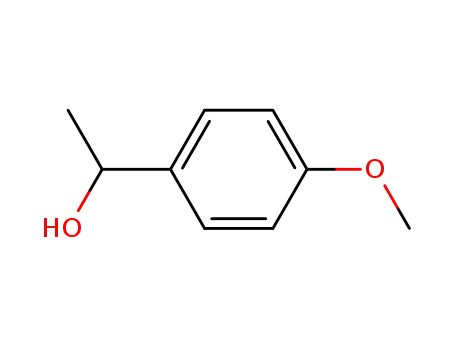
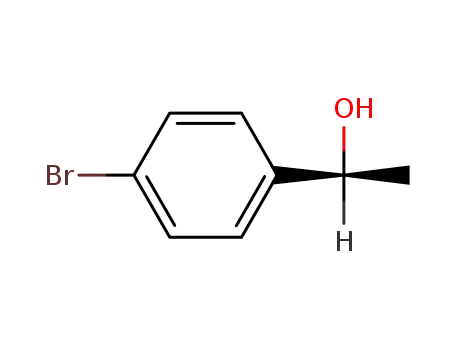
C1-Symmetric PNP Ligands for Manganese-Catalyzed Enantioselective Hydrogenation of Ketones: Reaction Scope and Enantioinduction Model
Zeng, Liyao,Yang, Huaxin,Zhao, Menglong,Wen, Jialin,Tucker, James H. R.,Zhang, Xumu
, p. 13794 - 13799 (2020)
A family of ferrocene-based chiral PNP l...
Iron achieves noble metal reactivity and selectivity: Highly reactive and enantioselective iron complexes as catalysts in the hydrosilylation of ketones
Bleith, Tim,Wadepohl, Hubert,Gade, Lutz H.
, p. 2456 - 2459 (2015)
Chiral iron alkyl and iron alkoxide comp...
Practical and efficient procedure for the in situ preparation of B-alkoxyoxazaborolidines. Enantioselective reduction of prochiral ketones
Ponzo, Viviana L.,Kaufman, Teodoro S.
, p. 495 - 496 (2000)
A new method for the in situ elaboration...
An efficient Ir(III) catalyst for the asymmetric transfer hydrogenation of ketones in neat water
Li, Xiaohong,Blacker, John,Houson, Ian,Wu, Xiaofeng,Xiao, Jianliang
, p. 1155 - 1160 (2006)
The chiral M-CsDPEN [M = Ru, Rh, Ir; CsD...
NH/π attraction: A Role in asymmetric hydrogenation of aromatic ketones with binap/1,2-diamine-ruthenium(II) complexes
Sandoval, Christian A.,Shi, Qixun,Liu, Shasha,Noyori, Ryoji
, p. 1221 - 1224 (2009)
-
Asymmetric hydrosilylation of ketones catalyzed by magnetically recoverable and reusable copper ferrite nanoparticles
Kantam, M. Lakshmi,Yadav, Jagjit,Laha, Soumi,Srinivas, Pottabathula,Sreedhar, Bojja,Figueras
, p. 4608 - 4611 (2009)
(Chemical Equation Presented) Herein we ...
Imidazolium ion tethered TsDPENs as efficient water-soluble ligands for rhodium catalyzed asymmetric transfer hydrogenation of aromatic ketones
Kang, Guowei,Lin, Silong,Shiwakoti, Atul,Ni, Bukuo
, p. 111 - 114 (2014)
An imidazolium ion tethered TsDPEN has b...
Optimisation, scope and advantages of the synthesis of chiral phenylethanols using whole seeds of Bauhinia variegata L. (Fabaceae) as a new and stereoselective bio-reducer of carbonyl compounds
Aimar, Mario L.,Bordón, Daniela L.,Cantero, Juan J.,Decarlini, María F.,Demmel, Gabriela I.,Rossi, Laura I.,Ruiz, Gustavo M.,Vázquez, Ana M.
, p. 1 - 15 (2020)
With the aim of finding new methods for ...
New air-stable iron catalyst for efficient dynamic kinetic resolution of secondary benzylic and aliphatic alcohols
Yang, Qiong,Zhang, Na,Liu, Mingke,Zhou, Shaolin
, p. 2487 - 2489 (2017)
We herein report a catalyst system for t...
Asymmetric palladium-catalyzed hydrosilylation of styrenes using efficient chiral spiro phosphoramidite ligands
Guo, Xun-Xiang,Xie, Jian-Hua,Hou, Guo-Hua,Shi, Wen-Jian,Wang, Li-Xin,Zhou, Qi-Lin
, p. 2231 - 2234 (2004)
Asymmetric hydrosilylation of styrene de...
Catalytic Asymmetric Addition of Organolithium Reagents to Aldehydes
Veguillas, Marcos,Solà, Ricard,Shaw, Luke,Maciá, Beatriz
, p. 1788 - 1794 (2016)
Herein we report an efficient catalytic ...
Enantioselective reduction of prochiral ketones employing sprouted Pisum sativa as biocatalyst
Yadav, Jhillu S.,Subba Reddy, Basi V.,Sreelakshmi, Chittamuru,Rao, Adari Bhaskar
, p. 1881 - 1885 (2009)
Sprouted green peas have been used for t...
Novel (+)-3-carene derivatives and their application in asymmetric synthesis
Roszkowski, Piotr,Malecki, Pawel,Maurin, Jan K.,Czarnocki, Zbigniew
, p. 569 - 574 (2015)
A simple synthetic procedure for the pre...
Chiral amplification based on enantioselective dual-phase distribution of a scalemic bisoxazolidine catalyst
Liu, Shuanglong,Wolf, Christian
, p. 2965 - 2968 (2007)
A readily available bisoxazolidine ligan...
Electronic effects of para-substitution on acetophenones in the reaction of rat liver 3α-hydroxysteroid dehydrogenase
Uwai, Koji,Konno, Noboru,Yasuta, Yuka,Takeshita, Mitsuhiro
, p. 1084 - 1089 (2008)
Stereoselective reductive metabolism of ...
Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum
Nakamura,Matsuda
, p. 8957 - 8964 (1998)
Aromatic ketones, β-keto esters, and sim...
New silica monolith bonded chiral (R)-γ butyrolactone for enantioselective micro high-performance liquid chromatography
Ghanem, Ashraf,Ikegami, Tohru,Tanaka, Nobuo
, p. 887 - 890 (2011)
A single low-molecular mass chiral selec...
New Chiral Ruthenium(II) Catalysts Containing 2,6-Bis(4′ -(R)-phenyloxazolin-2′-yl)pyridine (Ph-pybox) Ligands for Highly Enantioselective Transfer Hydrogenation of Ketones
Cuervo, Dario,Gamasa, M. Pilar,Gimeno, Jose
, p. 425 - 432 (2004)
Treatment of complex trans-[RuCl2(η2-C 2...
A simple protocol for the one pot synthesis of chiral secondary benzylic alcohols by catalytic enantioselective reduction of aromatic ketones
Ponzo, Viviana L.,Kaufman, Teodoro S.
, p. 1128 - 1130 (2002)
A simple and efficient protocol for the ...
Asymmetric transfer hydrogenation of ketones catalyzed by thermoregulated ionic liquid-regulating ruthenium complexes
Liu, Xuerui,Chen, Chen,Xiu, Yuhe,Chen, Angjun,Guo, Li,Zhang, Ran,Chen, Jizhong,Hou, Zhenshan
, p. 90 - 94 (2015)
A sulfonated chiral diamine ligand anion...
Synthesis, characterization, and organocatalytic application of chiral ionic liquids derived from (S,R)-noscapine
Kaur, Nirmaljeet,Chopra, Harish Kumar
, p. 26 - 31 (2018)
(S,R)-Noscapine, a phthalideisoquinoline...
Chiral-Mn(salen)-complex-catalyzed kinetic resolution of secondary alcohols in water
Sun, Wei,Wang, Hongwang,Xia, Chungu,Li, Jingwei,Zhao, Peiqing
, p. 1042 - 1044 (2003)
A mild, green method for enantioselectiv...
A Simple Biosystem for the High-Yielding Cascade Conversion of Racemic Alcohols to Enantiopure Amines
Li, Zhi,Tian, Kaiyuan
, p. 21745 - 21751 (2020)
The amination of racemic alcohols to pro...
Chiral bicyclic NHC/Ir complexes for catalytic asymmetric transfer hydrogenation of ketones
Yoshida, Kazuhiro,Kamimura, Takumi,Kuwabara, Hiroshi,Yanagisawa, Akira
, p. 15442 - 15445 (2015)
A diverse series of chiral bicyclic NHC/...
Control of enantioselectivity in the enzymatic reduction of halogenated acetophenone analogs by substituent positions and sizes
Koesoema, Afifa Ayu,Standley, Daron M.,Ohshima, Shusuke,Tamura, Mayumi,Matsuda, Tomoko
, (2020)
We utilized acetophenone reductase from ...
Catalytic enantioselective acyl transfer: the case for 4-PPY with a C-3 carboxamide peptide auxiliary based on synthesis and modelling studies
Cozett, Rudy E.,Venter, Gerhard A.,Gokada, Maheswara Rao,Hunter, Roger
, p. 10914 - 10925 (2016)
A series of 4-pyrrolidinopyridine (4-PPY...
Enantioselective acylation of alcohols catalyzed by lipase QL from Alcaligenes sp.: A predictive active site model for lipase QL to identify the faster reacting enantiomer of an alcohol in this acylation
Naemura,Murata,Tanaka,Yano,Hirose,Tobe
, p. 1581 - 1584 (1996)
Lipase QL-catalyzed acylation of seconda...
Copper-dipyridylphosphine-polymethylhydrosiloxane: A practical and effective system for the asymmetric catalytic hydrosilylation of ketones
Zhang, Xi-Chang,Wu, Fei-Fei,Li, Shijun,Zhou, Ji-Ning,Wu, Jing,Li, Ning,Fang, Wenjun,Lam, Kim Hung,Chan, Albert S. C.
, p. 1457 - 1462 (2011)
In the presence of the inexpensive and n...
Green synthesis of chiral aromatic alcohols with Lactobacillus kefiri P2 as a novel biocatalyst
Bayda?, Yasemin,Dertli, Enes,?ahin, Engin
, p. 1035 - 1045 (2020)
Biocatalytic reduction is a very importa...
Asymmetric reduction of aromatic ketones by the baker's yeast in organic solvent systems
Liu,Zhu,Sun,Xu
, p. 1521 - 1526 (2001)
The reduction of aromatic ketones to opt...
Chromoselective Photocatalysis Enables Stereocomplementary Biocatalytic Pathways**
Schmermund, Luca,Reischauer, Susanne,Bierbaumer, Sarah,Winkler, Christoph K.,Diaz-Rodriguez, Alba,Edwards, Lee J.,Kara, Selin,Mielke, Tamara,Cartwright, Jared,Grogan, Gideon,Pieber, Bartholom?us,Kroutil, Wolfgang
, p. 6965 - 6969 (2021)
Controlling the selectivity of a chemica...
Flower-like mesoporous silica: A bifunctionalized catalyst for rhodium-catalyzed asymmetric transfer hydrogenation of aromatic ketones in aqueous medium
Gao, Fei,Jin, Ronghua,Zhang, Dacheng,Liang, Quanxi,Ye, Qunqun,Liu, Guohua
, p. 2208 - 2214 (2013)
Functionalized flower-like mesoporous si...
Highly Enantioselective Production of Chiral Secondary Alcohols with Candida zeylanoides as a New Whole Cell Biocatalyst
?ahin, Engin,Dertli, Enes
, (2017)
The increasing demand for biocatalysts i...
Relative reactivity of substituted acetophenones in enantioselective biocatalytic reduction catalyzed by plant cells of Daucus carota and Petroselinum crispum
Chanysheva,Vorobyova,Zorin
, (2019)
We have examined enantioselective biored...
A ruthenium catalyst with simple triphenylphosphane for the enantioselective hydrogenation of aromatic ketones
Zhou, Han,Huang, Hanmin
, p. 2253 - 2257 (2013)
An efficient Ru catalyst constructed fro...
An efficient copper-aluminum hydrotalcite catalyst for asymmetric hydrosilylation of ketones at room temperature
Kantam, M. Lakshmi,Laha, Soumi,Yadav, Jagjit,Likhar, Pravin R.,Sreedhar,Jha, Shailendra,Bhargava, Suresh,Udayakiran,Jagadeesh
, p. 4391 - 4391 (2008)
-
Water-soluble arene ruthenium complexes containing a trans-1,2- diaminocyclohexane ligand as enantioselective transfer hydrogenation catalysts in aqueous solution
Canivet, Jerome,Labat, Gael,Stoeckli-Evans, Helen,Suess-Fink, Georg
, p. 4493 - 4500 (2005)
The cationic chloro complexes [(arene)Ru...
Biocompatible metal-assisted C-C cross-coupling combined with biocatalytic chiral reductions in a concurrent tandem cascade
Schaaf, Patricia,Bayer, Thomas,Koley, Moumita,Schnürch, Michael,Bornscheuer, Uwe T.,Rudroff, Florian,Mihovilovic, Marko D.
, p. 12978 - 12981 (2018)
In this study, we present a concurrent c...
In situ measurement of the enantiomeric excess of alcohols and amines under asymmetric reduction reaction by 1H NMR
Ye, Xiaoxia,Lei, Xinxiang,Chen, Zhenfei,Zhang, Lixue,Zhang, Anjiang
, p. 3238 - 3241 (2010)
(Figure Presented) 1H NMR, in situ, dete...
Reduction of various ketones by red algae
Utsukihara, Takamitsu,Misumi, Osami,Kato, Nakahide,Kuroiwa, Tsuneyoshi,Horiuchi, C. Akira
, p. 1179 - 1185 (2006)
The reduction of acetophenone derivative...
Highly recoverable organoruthenium-functionalized mesoporous silica boosts aqueous asymmetric transfer hydrogenation reaction
Liu, Rui,Cheng, Tanyu,Kong, Lingyu,Chen, Chen,Liu, Guohua,Li, Hexing
, p. 55 - 61 (2013)
Exploring functionalized mesoporous sili...
An efficient catalyst system for the asymmetric transfer hydrogenation of ketones: Remarkably broad substrate scope
Reetz, Manfred T.,Li, Xiaoguang
, p. 1044 - 1045 (2006)
A BINOL-derived diphosphonite having a x...
Asymmetric bioreduction of p-haloacetophenones by Mucor
Zhang, Zuohui,Liu, Xiongmin,Ma, Li,Zhuo, Meifang,Shen, Fang
, p. 323 - 328 (2013)
A number of p-haloacetophenones were asy...
Enantioselective reduction of bromo- and methoxy-acetophenone derivatives using carrot and celeriac enzymatic system
Maczka, Wanda K.,Mironowicz, Agnieszka
, p. 1965 - 1967 (2004)
The enantioselective reductions of the c...
Water-soluble chiral monosulfonamide-cyclohexane-1,2-diamine-RhCp* complex and its application in the asymmetric transfer hydrogenation (ATH) of ketones
Cortez, Norma A.,Aguirre, Gerardo,Parra-Hake, Miguel,Somanathan, Ratnasamy
, p. 4335 - 4338 (2007)
Monosulfonamide ligands with heteroatom/...
Reversible, short α-peptide assembly for controlled capture and selective release of enantiomers
Chen, Xi,He, Ying,Kim, Yongju,Lee, Myongsoo
, p. 5773 - 5776 (2016)
Although significant progress has been a...
Reduction of 4-Substituted Acetophenones by Yeast
Eichberger, Guenter,Faber, Kurt,Griengl, Herfried
, p. 1233 - 1236 (1985)
The velocity of reduction of 4-substitut...
Mesoporous SBA-15-supported chiral catalysts: preparation, characterization and asymmetric catalysis
Liu, Guohua,Liu, Mouming,Sun, Yunqiang,Wang, Jianyao,Sun, Chuanshou,Li, Hexing
, p. 240 - 246 (2009)
Two mesoporous silica-supported chiral R...
Highly enantiomeric reduction of acetophenone and its derivatives by locally isolated Rhodotorula glutinis
Zilbeyaz, Kani,Kurbanoglu, Esabi B.
, p. 849 - 854 (2010)
Ninety isolates of microorganisms belong...
Aerobic oxidative kinetic resolution of racemic alcohols with bidentate ligand-binding Ru(salen) complex as catalyst
Nakamura, Yukie,Egami, Hiromichi,Matsumoto, Kazuhiro,Uchida, Tatsuya,Katsuki, Tsutomu
, p. 6383 - 6387 (2007)
Chiral Ru(salen)(nitrosyl) complex 1 is ...
Exploration of highly electron-rich manganese complexes in enantioselective oxidation catalysis; A focus on enantioselective benzylic oxidation
Klein Gebbink, Robertus J. M.,Li, Fanshi,Lutz, Martin,Masferrer-Rius, Eduard
, p. 7751 - 7763 (2021/12/13)
The direct enantioselective hydroxylatio...
Chitosan as a chiral ligand and organocatalyst: Preparation conditions-property-catalytic performance relationships
Kolcsár, Vanessza Judit,Sz?ll?si, Gy?rgy
, p. 7652 - 7666 (2021/12/13)
Chitosan is an abundant and renewable ch...
Chiral Iron(II)-Catalysts within Valinol-Grafted Metal-Organic Frameworks for Enantioselective Reduction of Ketones
Akhtar, Naved,Antil, Neha,Begum, Wahida,Chauhan, Manav,Kumar, Ajay,Manna, Kuntal,Newar, Rajashree
, p. 10450 - 10459 (2021/08/31)
The development of highly efficient and ...
Amino Acid-Functionalized Metal-Organic Frameworks for Asymmetric Base–Metal Catalysis
Newar, Rajashree,Akhtar, Naved,Antil, Neha,Kumar, Ajay,Shukla, Sakshi,Begum, Wahida,Manna, Kuntal
supporting information, p. 10964 - 10970 (2021/03/29)
We report a strategy to develop heteroge...